CMS Publishes Two New Reports on GADCS Website
 |
| Dear Ground Ambulance Providers and Suppliers,
We wanted you to be aware that we posted 2 reports on our Medicare Ground Ambulance Data Collection System website under Reports: |
 |
| Dear Ground Ambulance Providers and Suppliers,
We wanted you to be aware that we posted 2 reports on our Medicare Ground Ambulance Data Collection System website under Reports: |
The letter to the editor below was submitted to The Guardian on July 23, following the July 21 publication of the article referenced.
To the Editor of The Guardian,
On July 21, The Guardian published Jessica Glenza’s “Plan to end exorbitant ‘surprise’ ambulance bills heads to Congress.” The inflammatory title and lack of context do no justice to the years of bipartisan collaborative effort leading to the forthcoming report to Congress. As a member of the Advisory Committee on Ground Ambulance and Patient Billing (GAPB) and Immediate Past President of the American Ambulance Association, I believe it is critical to set the record straight.
It is essential to understand that EMS directly bills patients instead of insurers only as a last resort. Sadly, as a frequent entry point to healthcare, EMS often faces the unenviable task of educating people about their limited insurance coverage or high deductibles, both of which are out of our control. As mobile healthcare is entirely decentralized in the United States, it is often unfeasible for small or volunteer-staffed ambulance services to negotiate sustainable in-network rates with dozens of insurance plans. The GAPB Advisory Committee’s recommendations seek to remedy this foundational disconnect between patients, EMS providers, and health plans.
The article notes that some EMS providers are owned by private equity, but overlooks that the vast majority of ambulance services in the United States are small, often conducting only a few dozen patient transports per day. These community-based services—some of which are the sole healthcare provider for miles—face skyrocketing costs for wages, fuel, and medical supplies that threaten their ability to keep their doors open. The collaborative work of the GAPB Advisory Committee sought to address these challenges by proposing recommendations that, if adopted by Congress, would help alleviate these financial pressures while also enhancing patient protection from surprise insurance denials.
The article implies that Patricia Kelmar was the only representative of the public interest on the panel. In fact, another Committee participant was explicitly appointed to represent patient advocacy groups, and as healthcare providers, EMS professionals and physicians consistently advocate for our patients’ well-being. The committee’s composition, as established by Congress within the No Surprises Act, was intentionally diverse and included stakeholders ranging from physicians to elected officials to insurance providers to ensure balance.
Additionally, it is important to clarify that the Health Affairs research cited in the article does not provide data on actual balance bills received by patients. Rather, it roughly estimates only potential balance bills as calculated based on a flawed estimation process. Even if we were to accept Health Affairs estimates as fact, the average balance bill calculated according to their methods would be just a few hundred dollars. This is far from the sole and extreme outlier bill cited in the piece. This distinction is critical as it underscores the need for data-driven policy decisions based on real-world evidence rather than projections and one-off examples.
Finally, the piece misses entirely the largest challenge with the Committee’s recommendations and their potential adoption by Congress. Based on longstanding legal precedent, ERISA plans, which cover about half of Americans through their employers, would not be bound by any legislation drafted based on our report. In Washington state and elsewhere, innovative “opt-in” clauses enable ERISA plans to voluntarily comply with state regulation. We encourage this and hope to see it replicated throughout the nation.
People become first responders because they have a passion for caring for others, and our communities trust them to do just that—24/7. Our Committee’s report to Congress includes 14 key recommendations designed to improve transparency, ensure fair reimbursement rates, and ultimately protect patients by strengthening state and local control. If these recommendations are adopted, they will help remove patients from the middle of billing disputes, allowing EMS providers to focus on our primary mission: delivering life-saving and life-sustaining healthcare around the clock.
For a detailed understanding of our recommendations and the Committee’s work, I encourage reading the full GAPB Advisory Committee report when it becomes available later this summer.
Shawn Baird
Immediate Past President, American Ambulance Association
Member, Advisory Committee on Ground Ambulance and Patient Billing
Portland, Oregon
Please either or Join!
|
||||||||||||||||
|
Please either or Join!
From the OSHA Website on June 4, 2024
NEW Update: The deadline for comment submission has been extended a second time to July 22, 2024.
The Emergency Response proposed rule is here!
OSHA is happy to announce that the Emergency Response proposed rule has been published in the Federal Register and is now available for viewing.
OSHA welcomes and encourages the submission of public comments in response to this proposed rule. To allow additional time for those individuals interested in creating and submitting a comment, OSHA will be further extending the window for comment submission. The comment period now ends on July 22, 2024.
Comments can be submitted to the Emergency Response Docket at https://www.regulations.gov/docket/OSHA-2007-0073.
OSHA will also be hosting a public hearing, the date of which has yet to be determined. To ensure access to the hearing for all interested members of the public, remote access will be provided.
Additional information on OSHA’s rulemaking process and how stakeholders can participate is available at https://www.osha.gov/laws-regs/rulemakingprocess.
Please either or Join!
Please either or Join!
The next CMS Ambulance Open Door Forum scheduled for:
Date: Thursday, April 11, 2024
Start Time: 2:00pm-3:00pm PM Eastern Time (ET);
Please dial-in at least 15 minutes before call start time.
Conference Leaders: Jill Darling, Maria Durham
**This Agenda is Subject to Change**
I. Opening Remarks
Chair- Maria Durham, Director, Division of Data Analysis and Market Based Pricing
Moderator – Jill Darling (Office of Communications)
II. Announcements & Updates
1. Medicare Ground Ambulance Data Collection System (GADCS)
III. Open Q&A
**DATE IS SUBJECT TO CHANGE**
Next Ambulance Open Door Forum: TBA
ODF email: AMBULANCEODF@cms.hhs.gov
———————————————————————
This Open Door Forum is open to everyone, but if you are a member of the Press,
you may listen in but please refrain from asking questions during the Q & A portion of
the call. If you have inquiries, please contact CMS at Press@cms.hhs.gov. Thank
you.
NEW and UPDATED Open Door Forum Participation Instructions:
This call will be a Zoom webinar with registration and login instructions below.
Register in advance for this webinar:
https://cms.zoomgov.com/webinar/register/WN_vfsU5LSKR3atiW9T_AhrDg
Meeting ID: 160 823 4591
Passcode: 200020
After registering, you will receive a confirmation email containing information about
joining the webinar. You may also add the webinar to your calendar using the dropdown arrow on the “Webinar Registration Approved” webpage after registering.’
Although the ODFs are now a Zoom webinar, we will only use the audio function, no need for cameras to be on.
For ODF schedule updates and E-Mailing List registration, visit our website at http://www.cms.gov/OpenDoorForums/.
Were you unable to attend the recent Ambulance ODF call? We encourage you to visit our CMS Podcasts and Transcript webpage where you can listen and view the most recent Ambulance ODF call. The webinar recording and transcript will be posted to: https://www.cms.gov/Outreach-andEducation/Outreach/OpenDoorForums/PodcastAndTranscripts.html.
CMS provides free auxiliary aids and services including information in accessible formats. Click here for more information. This will point partners to our CMS.gov version of the “Accessibility & Nondiscrimination notice” page. Thank you.
|
Please either or Join!
From EMS.gov on January 24, 2024
|
|
|
|
Final rule modernizes the health care system and reduces patient and provider burden by streamlining the prior authorization process
As part of the Biden-Harris Administration’s ongoing commitment to increasing health data exchange and strengthening access to care, the Centers for Medicare & Medicaid Services (CMS) finalized the CMS Interoperability and Prior Authorization Final Rule (CMS-0057-F) today. The rule sets requirements for Medicare Advantage (MA) organizations, Medicaid and the Children’s Health Insurance Program (CHIP) fee-for-service (FFS) programs, Medicaid managed care plans, CHIP managed care entities, and issuers of Qualified Health Plans (QHPs) offered on the Federally-Facilitated Exchanges (FFEs), (collectively “impacted payers”), to improve the electronic exchange of health information and prior authorization processes for medical items and services. Together, these policies will improve prior authorization processes and reduce burden on patients, providers, and payers, resulting in approximately $15 billion of estimated savings over ten years.
“When a doctor says a patient needs a procedure, it is essential that it happens in a timely manner,” said HHS Secretary Xavier Becerra. “Too many Americans are left in limbo, waiting for approval from their insurance company. Today the Biden-Harris Administration is announcing strong action that will shorten these wait times by streamlining and better digitizing the approval process.”
“CMS is committed to breaking down barriers in the health care system to make it easier for doctors and nurses to provide the care that people need to stay healthy,” said CMS Administrator Chiquita Brooks-LaSure. “Increasing efficiency and enabling health care data to flow freely and securely between patients, providers, and payers and streamlining prior authorization processes supports better health outcomes and a better health care experience for all.”
While prior authorization can help ensure medical care is necessary and appropriate, it can sometimes be an obstacle to necessary patient care when providers must navigate complex and widely varying payer requirements or face long waits for prior authorization decisions. This final rule establishes requirements for certain payers to streamline the prior authorization process and complements the Medicare Advantage requirements finalized in the Contract Year (CY) 2024 MA and Part D final rule, which add continuity of care requirements and reduce disruptions for beneficiaries. Beginning primarily in 2026, impacted payers (not including QHP issuers on the FFEs) will be required to send prior authorization decisions within 72 hours for expedited (i.e., urgent) requests and seven calendar days for standard (i.e., non-urgent) requests for medical items and services. For some payers, this new timeframe for standard requests cuts current decision timeframes in half. The rule also requires all impacted payers to include a specific reason for denying a prior authorization request, which will help facilitate resubmission of the request or an appeal when needed. Finally, impacted payers will be required to publicly report prior authorization metrics, similar to the metrics Medicare FFS already makes available.
The rule also requires impacted payers to implement a Health Level 7 (HL7®) Fast Healthcare Interoperability Resources (FHIR®) Prior Authorization application programming interface (API), which can be used to facilitate a more efficient electronic prior authorization process between providers and payers by automating the end-to-end prior authorization process. Medicare FFS has already implemented an electronic prior authorization API, demonstrating the efficiencies other payers could realize by implementing such an API. Together, these new requirements for the prior authorization process will reduce administrative burden on the healthcare workforce, empower clinicians to spend more time providing direct care to their patients, and prevent avoidable delays in care for patients.
In response to feedback received on multiple rules and extensive stakeholder outreach HHS will be announcing the use of enforcement discretion for the Health Insurance Portability and Accountability Act of 1996 (HIPAA) X12 278 prior authorization transaction standard to further promote efficiency in the prior authorization process. Covered entities that implement an all-FHIR-based Prior Authorization API pursuant to the CMS Interoperability and Prior Authorization Final Rule (CMS-0057-F) who do not use the X12 278 standard as part of their API implementation will not be enforced against under HIPAA Administrative Simplification, thus allowing limited flexibility for covered entities to use a FHIR-only or FHIR and X12 combination API to meet the requirements of the CMS Interoperability and Prior Authorization final rule. Covered entities may also choose to make available an X12-only prior authorization transaction. HHS will continue to evaluate the HIPAA prior authorization transaction standards for future rulemaking.
CMS is also finalizing API requirements to increase health data exchange and foster a more efficient health care system for all. CMS values public input and considered the comments submitted by the public, including patients, providers, and payers, in finalizing the rule. Informed by these public comments, CMS is delaying the dates for compliance with the API policies from generally January 1, 2026, to January 1, 2027. In addition to the Prior Authorization API, beginning January 2027, impacted payers will be required to expand their current Patient Access API to include information about prior authorizations and to implement a Provider Access API that providers can use to retrieve their patients’ claims, encounter, clinical, and prior authorization data. Also informed by public comments on previous payer-to-payer data exchange policies, we are requiring impacted payers to exchange, with a patient’s permission, most of those same data using a Payer-to-Payer FHIR API when a patient moves between payers or has multiple concurrent payers.
Finally, the rule also adds a new Electronic Prior Authorization measure for eligible clinicians under the Merit-based Incentive Payment System (MIPS) Promoting Interoperability performance category and eligible hospitals and critical access hospitals (CAHs) in the Medicare Promoting Interoperability Program to report their use of payers’ Prior Authorization APIs to submit an electronic prior authorization request. Together, these policies will help to create a more efficient prior authorization process and support better access to health information and timely, high-quality care.
The final rule is available to review here: https://www.cms.gov/files/document/cms-0057-f.pdf.
The fact sheet for this final rule is available here: https://www.cms.gov/newsroom/fact-sheets/cms-interoperability-and-prior-authorization-final-rule-cms-0057-f.
###
The US Fire Administration (USFA) and Fire Safety Research Institute (FSRI) have announced the commencement of a national engagement period for the National Emergency Response Information System (NERIS) Draft Data Framework. This period will end on January 19, 2024.
The EMS community is invited to offer feedback on the Draft Core NERIS Data Framework, which includes essential data schemas crucial for NERIS operations, designed to provide the EMS and fire community with the necessary data and tools for improved decision-making and enhanced incident preparedness. These schemas include:
Access the Draft NERIS Data Framework and submit feedback by January 19, 2024. To submit feedback, access the feedback submission form below after reviewing the Framework.
|
For accessibility requests or further assistance, please contact NERIS@ul.org.
Please either or Join!
Individuals with private health insurance can receive “surprise bills” for the difference between what a provider charged and what their insurance paid.
A 2021 law prohibits surprise billing for some services, and directed the Departments of Health and Human Services, Labor, and Treasury to give providers and insurers a forum to resolve disputes about how much insurers should pay for out-of-network care.
But the rollout has been challenging. As of June 2023, over 490,000 disputes have been submitted, a much larger number than anticipated by the agencies.
And 61% of the disputes are unresolved as of June 2023.

The No Surprises Act directed the departments of Health and Human Services (HHS), Labor, and Treasury to establish a federal independent dispute resolution process. The process, which was effective April 2022, is a voluntary forum for health care providers and health insurance issuers to resolve disputes about how much should be paid for out-of-network care. The payment determinations are made by certified dispute resolution entities, which serve as arbiters. The Centers for Medicare & Medicaid Services (CMS)—an agency within HHS—administers the independent dispute resolution process.
The three departments reported that parties submitted nearly 490,000 disputes from April 2022 through June 2023. About 61 percent of these disputes remained unresolved as of June 2023. According to officials from the departments, a primary cause of the large number of unresolved disputes is the complexity of determining whether disputes are eligible for the process.
Number of Out-of-Network Disputes in the Federal Independent Dispute Resolution Process by Calendar Quarter, April 15, 2022—June 30, 2023
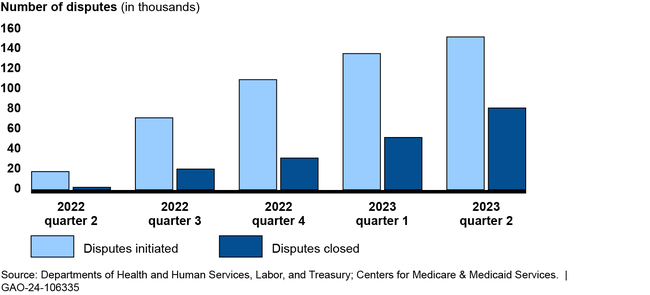
The groups GAO interviewed described a challenging roll out of the independent dispute resolution process, including a higher-than-expected dispute volume. For example, the departments anticipated about 22,000 disputes in 2022, but received nearly 490,000 through June 2023. Four groups told GAO the departments did not account for the experience of states with similar processes when making the estimate. Disputing parties and certified entities also described the broader effects of those challenges, such as backlogs resulting in delays in payment determinations. The departments have taken some actions to address challenges, such as conducting pre-eligibility reviews on submitted disputes.
To address concerns from insurers and providers, CMS and Labor look into complaints; however, stakeholder groups expressed concern with what they describe as a lack of response to submitted complaints. The departments reported limited ability to increase enforcement efforts due to budget constraints. HHS has requested a budget increase for the process, and the departments are revisiting the administrative fee amount, which is intended to cover the costs of the process, and plan to issue updated program rules.
About two thirds of individuals in the United States receive their health coverage through private health plans. Balance billing is when insured patients receive a bill from a health care provider for the difference between the amount charged and the payment received from the health insurance issuer. An unexpected balance bill is referred to as a “surprise bill” and may create a financial strain for patients. For individuals with private health insurance, the No Surprises Act prohibits providers from balance billing in certain circumstances and directed the three departments to establish the federal independent dispute resolution process.
The Consolidated Appropriations Act, 2021, includes a provision for GAO to review the federal independent dispute resolution process. This report describes (1) the number and types of disputes submitted between April 2022 and June 2023, and the status of their resolution; (2) selected stakeholders’ experiences with the process, and agency actions to address challenges; and (3) how federal agencies oversee the process.
GAO reviewed published reports, relevant federal laws, regulations, and guidance; and interviewed officials from CMS and Labor. GAO also interviewed five selected health care providers or their representatives, which accounted for nearly half of all submitted disputes as of December 2022. In addition, GAO interviewed three issuers, three certified entities that arbitrate the disputes, and 10 stakeholder groups.
For more information, contact John E. Dicken at (202) 512-7114 or dickenj@gao.gov.
Please either or Join!
NHTSA published a request for information on October 13, 2023, seeking comments from all sources (public, private, government, academic, professional, public interest groups, and other interested parties) on the planned re-envisioning of the 2000 EMS Education Agenda for the Future: A Systems Approach. Due to the limited comments received and some informal feedback indicating that the initial comment period was too short, NHTSA is announcing the reopening of the comment period for the RFI in order to solicit additional comments and request responses to specific questions provided in the document. The comment period for the RFI was originally scheduled to end on October 31, 2023. It will now be reopened and will end on March 31, 2024.
The comment period for the RFI published on October 13, 2023 at 88 FR 71081 is reopened and extended to March 31, 2024.
Clary Mole, EMS Specialist, National Highway Traffic Safety Administration, U.S. Department of Transportation is available by phone at (202) 868–3275 or by email at Clary.Mole@dot.gov.
Comments must be submitted by one of the following methods:
• Federal eRulemaking Portal: go to http://www.regulations.gov. Follow the online instructions for submitting comments.
• Mail: Docket Management Facility, M–30, U.S. Department of Transportation, West Building, Ground Floor, Rm. W12–140, 1200 New Jersey Avenue SE, Washington, DC 20590.
• Hand Delivery or Courier: West Building Ground Floor, Room W12–140, 1200 New Jersey Avenue SE, between 9 a.m. and 5 p.m. Eastern Time, Monday through Friday, except Federal holidays. To be sure someone is there to help you, please call (202) 366–9322 before coming.
Regardless of how you submit your comments, you must include the docket number identified in the heading of this document.
Note that all comments received, including any personal information provided, will be posted without change to http://www.regulations.gov. Please see the “Privacy Act” heading below.
You may call the Docket Management Facility at (202) 366–9322. For access to the docket to read background documents or comments received, go to http://www.regulations.gov or the street address listed above. We will continue to file relevant information in the docket as it becomes available. To be sure someone is there to help you, please call (202) 366–9322 before coming. We will continue to file relevant information in the Docket as it becomes available.
Privacy Act: In accordance with 5 U.S.C. 553(c), DOT solicits comments from the public to inform its decision-making process. DOT posts these comments, without edit, including any personal information the commenter provides, to http://www.regulations.gov, as described in the system of records notice (DOT/ALL–14 FDMS), which can be reviewed at https://www.transportation.gov/privacy. Anyone is able to search the electronic form of all comments received into any of our dockets by the name of the individual submitting the comment (or signing the comment, if submitted on behalf of an association, business, labor union, etc.). You may review DOT’s complete Privacy Act Statement in the Federal Register published on April 11, 2000 ( 65 FR 19477–78 ).
On October 13, 2023, NHTSA published a RFI to obtain public comments to inform EMS Education Agenda 2050, and request responses to specific questions provided in this document. For convenience purposes, NHTSA is republishing introductory information, background materials and questions from its RFI in this notice.
In 2012, the National EMS Advisory Council (NEMSAC) convened a national roundtable meeting on EMS Education Agenda for the Future: A Systems Approach. In a 2014 report on these proceedings, NEMSAC advised that stakeholders at the State and local level had just begun to experience the full impact of the evolution toward a national integrated system of education for EMS personnel. While stakeholders were reticent to move forward with a new education agenda, they did provide feedback about themes that should be considered in the future publication. From the feedback collected at the meeting, NEMSAC developed recommendations to be used in the eventual re-envision of the agenda for EMS. These recommendations are summarized below:
• Mobile Integrated Healthcare has received considerable attention from the EMS Community. This and other alternative community-based healthcare delivery models (of the future) should evoke an expanded foundational knowledge and critical thinking capabilities that will poise future EMS practitioners to be able to evolve with the changing healthcare system or rapidly adjust to emerging healthcare crises.
In the 10 years since NEMSAC’s roundtable meeting, the national EMS education system continued to evolve—especially during the COVID–19 pandemic. In late 2021, the Federal Interagency Committee on EMS (FICEMS) began sponsoring listening sessions to inform a consensus-driven, national report entitled, FICEMS: EMS and 911 COVID–19 Response White Paper. This publication cited challenges and solutions collected during stakeholder listening sessions for the EMS education system. Among the challenges, EMS education stakeholders cited scarcity (in some cases deficits) in resources for education, rigidity of curriculum delivery modalities, the increased employer demands on students, and inconsistent or delayed responses to the needs of the national EMS education system as major contributors that led to the breakdown in the EMS workforce pipeline.
Prior to the COVID–19 pandemic, NHTSA published EMS Agenda 2050: A People-centered Vision for the Future of EMS (Agenda 2050). This collaborative project set a vision for a people-centered EMS systems that serves every individual in every community across the Nation. Later this year, NHTSA and its partners will begin a new project to develop EMS Education Agenda 2050. This project will not replace but build upon the achievements of the 2000 EMS Education Agenda for the Future: A Systems Approach to lead a national conversation around the future vision for EMS Education and EMS as a profession.
NHTSA, in partnership with Health Resources and Services Administration, published EMS Education Agenda for the Future: A Systems Approach ( Education Agenda ) in 2000. This document was founded on the broad national EMS education system concepts introduced in the EMS Agenda for the Future (1996). The Education Agenda described a consensus vision of an EMS education system with a high degree of structure, coordination, and interdependence. It proposed a less prescriptive system that offered educators flexibility in creating a student-centered learning environment and a process for accommodating future advancements in technology and medicine. The proposed system maximized efficiency, consistency in instructional quality, and entry level graduate competency by prescribing a high degree of structure, coordination, and interdependence. To achieve this vision, the education system of the future centered on five integrated primary components:
After the Education Agenda was published, stakeholders began implementing their respective integrated system components. Almost 25 years later, the national EMS education system has successfully evolved into one that exemplifies both consistency and flexibility. System interdependencies have helped to avoid duplication of effort in curriculum and education program development, evaluating the minimum competencies of graduates, certification and licensing processes, and facilitation of practitioner reciprocity.
In 2020, the EMS education system interdependencies modernized by the Education Agenda were tested. Challenges presented by the COVID–19 pandemic forced a variety of adaptations. Traditional education programs reported a lag in students’ capabilities of achieving the programmatic competencies requirements for graduation. The lag was attributed to a variety of causes including a focus on pandemic response activities over training and education, employer demands on working students, and the rigidity of in-person, classroom-based education delivery models. After the majority of programs adjusted to the challenges, lags in graduation were cured, and students achieved programmatic competencies at rates similar to those pre-pandemic. The response to the pandemic did not impact education programs only. The impact to EMS agency daily operations was felt as well. During the COVID pandemic, agencies experienced increases in EMS activation and response rates which created additional stressors for student EMS practitioners already working in a high stress job environment but also enrolled in an EMS education program. These stressors were a major contributor to a migration of practitioners away from the EMS workforce. Agencies and organizational stakeholders asserted that it could be education program graduation requirements causing breakdown in the workforce pipeline; however, there were no observed decreases in graduation or certification testing rates. These observations prompt two questions: If graduation and certification testing rates have remained unchanged, why have agencies reported recruitment and retention issues? If graduates are not entering the EMS workforce, where are they finding jobs?
With agencies experiencing increased demand and a deficiency in qualified EMS practitioners to respond to it, service delivery models had to evolve. To bridge the gap in community-based care resources, community paramedicine and mobile integrated healthcare (CP–MIH) service delivery models increased in prevalence, and improvised training programs were used to close new job-specific competency gaps among existing EMS practitioners and individuals in training. Other themes brought to the forefront during the pandemic include addressing healthcare disparities; the use of EMS data as a tool for surveillance and nationwide quality of care improvements; and a greater value to having an EMS workforce that is not only equitable, inclusive, and accessible, but as diverse as the community it serves. These themes, evolving service delivery models, and the subsequent evolution of competencies needed by practitioners suggest that it is time for NHTSA to gather our partners to begin a new conversation about the future of EMS Education and EMS as a profession in the United States.
Responses to the following questions are requested to help plan the revision of the Education Agenda. Please be as specific as possible and as appropriate please provide references.
1. What are the most critical issues facing EMS education system that should be addressed in the revision of the EMS Education Agenda ? Please provide specific examples.
2. What progress has been made in implementing the EMS Education Agenda since 2000?
3. How have you used EMS Education Agenda ? Please provide specific examples.
4. As an EMS Stakeholder, how might a revised EMS Education Agenda be most useful to you?
5. What significant changes have occurred in the EMS education system at the national, Federal, State, and local levels since 2000?
6. What significant changes will impact the EMS education system in the next 25 years?
7. How might the revised EMS Education Agenda contribute to enhanced EMS for children?
8. How might the revised EMS Education Agenda support and/or promote data-driven and evidence-based improvements in EMS education systems and EMS practitioner practice?
9. How could the revised EMS Education Agenda enhance collaboration among EMS systems, health care providers and facilities, public safety answering points, public health, public safety, emergency management, insurers, and others?
10. How could the revised EMS Education Agenda be used to promote community sustainability and resilience?
11. How could the revised EMS Education Agenda contribute to improved coordination for disaster response, recovery, preparedness, and mitigation?
12. How could the revised EMS Education Agenda enhance the exchange of evidence-based practices between national, Federal (and military), State, and local levels?
13. How could the revised EMS Education Agenda support the seamless and unimpeded transfer of military EMS personnel to roles as civilian EMS providers?
14. How could the revised EMS Education Agenda support interstate credentialing of EMS personnel?
15. How could the revised EMS Education Agenda support improved patient outcomes in rural and frontier communities?
16. How could the revised EMS Education Agenda lead to improved EMS systems in tribal communities?
17. How could the revised EMS Education Agenda promote a culture of safety among EMS personnel, agencies, and organizations?
18. Are there additional EMS attributes that should be included in the revised EMS Education Agenda ? If so, please provide an explanation for why these additional EMS attributes should be included.
19. Are there EMS attributes in the 2000 EMS Education Agenda that should be eliminated from the revised edition? If so, please provide an explanation for why these EMS attributes should be eliminated.
20. What are your suggestions for the process that should be used in revising the EMS Education Agenda ?
21. What specific agencies/organizations/entities are essential to involve, in a revision of the EMS Education Agenda ?
22. Do you have any additional comments regarding the revision of the EMS Education Agenda ?
(Authority: 23 U.S.C. 403(b)(1)(A)(iv); 49 CFR 1.95; 501.8)
Issued in Washington, DC.
Please either or Join!